

The development of effective drugs for Central Nervous System (CNS) disorders remains a significant challenge due to the complexities of the human brain and the limitations of traditional drug delivery methods. Stereotaxic animal models combined with intracerebroventricular (ICV) drug administration have emerged as an essential tool for understanding the pharmacokinetics and pharmacodynamics of potential CNS drug candidates.
This white paper outlines the application of a stereotaxic animal model with ICV delivery to enhance CNS drug delivery, focusing on methotrexate (MTX), a widely used immunosuppressive and chemotherapy agent, for the treatment of rheumatoid arthritis and neoplastic diseases, such as Acute Lymphoblastic Leukemia (ALL) and non-Hodgkin lymphoma. By establishing this model, we have gained valuable insights into methotrexate’s distribution across various brain regions and plasma, highlighting the potential of ICV delivery to optimize drug penetration, reduce systemic toxicity, and improve therapeutic outcomes for low-permeability CNS drugs.
Overview of CNS Drug Development
Developing therapeutics for CNS disorders is hindered by multiple challenges, primarily due to:
Importance of Animal Models
Animal models play a crucial role in evaluating the efficacy, pharmacokinetics, and pharmacodynamics of CNS drugs.
Among these, stereotaxic models enable precise drug administration, thereby facilitating accurate assessments of drug distribution in the brain.
Overview of Methotrexate
Relevance in CNS Drug Development
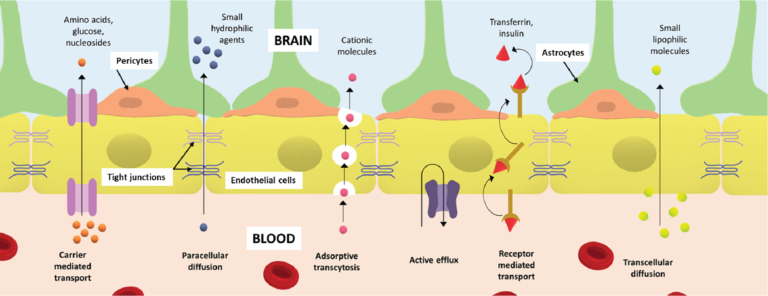
Figure 1: Blood brain barrier (BBB) depicting various types of transport systems across it.
Stereotaxic Surgery for CNS Drug Delivery
Intracerebroventricular (ICV) Administration:
Objective
To assess the effectiveness of combining ICV administration with stereotaxic surgery for targeted delivery of methotrexate to specific brain regions, improving drug distribution and therapeutic outcomes for CNS lymphoma treatment.
Hypothesis
The combination of ICV administration and stereotaxic surgery will enhance MTX delivery to the brain, improving bioavailability and reducing systemic toxicity.
Materials and Methods
ICV Injection Procedure
1. Preparation:
2. Positioning:
3. Surgical Procedure:

Figure 2: Stereotaxic apparatus.
MTX Distribution in Brain Regions
MTX Plasma Concentrations
Drug Distribution
Stereotaxic Surgery
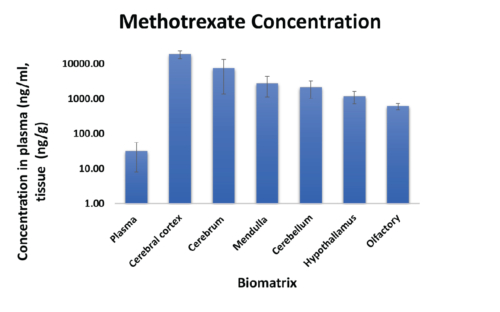
Figure 3: Methotrexate concentration in plasma and brain parenchyma.
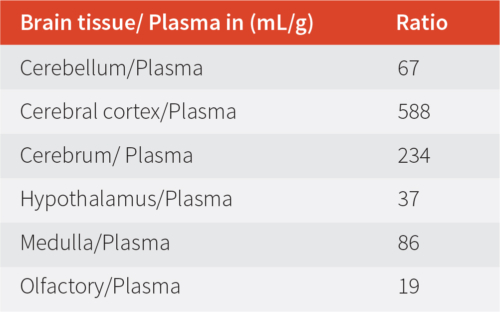
Table 1: Brain tissue and plasma ratio.
Key Findings
Implications for Drug Development
Limitations & Recommendations
