

Technology transfer of a drug substance is a multifaceted activity with inherent technical, regulatory, and quality risks that must be addressed to ensure a successful outcome. At Aragen, we specialize in technology transfer services that ensure smooth and efficient transitions of drug substance development across all phases—from early discovery to commercial-scale production. Our team combines deep expertise, advanced infrastructure, and a commitment to quality to facilitate the seamless transfer of processes, technologies, and knowledge throughout your project’s lifecycle.
We offer a proven and adaptable approach to technology transfer, whether for scale-up or transitioning to a different facility. Our experts meticulously assess the inherent complexity of your project, ensuring that our transfer scope and strategy are perfectly aligned with your specific needs. From small-scale development to large-scale commercial manufacturing, our goal is to ensure the efficiency and success of the transfer process at every phase.
Structured to support manufacturing operations in an FFS-based partnership model, we have access to a large-scale commercial cGMP production plant, a cGMP pilot plant, and cGMP kilo laboratories. This infrastructure ensures that we can deliver processes that meet both GMP and non-GMP requirements on time and with the appropriate quality, while minimizing risks and optimizing process scalability.
With a focus on reducing delays, maintaining product integrity, and meeting global regulatory standards, we guide your development through each critical transition, ensuring that your path to market is as smooth, efficient, and compliant as possible.
Our Approach to Technology Transfer
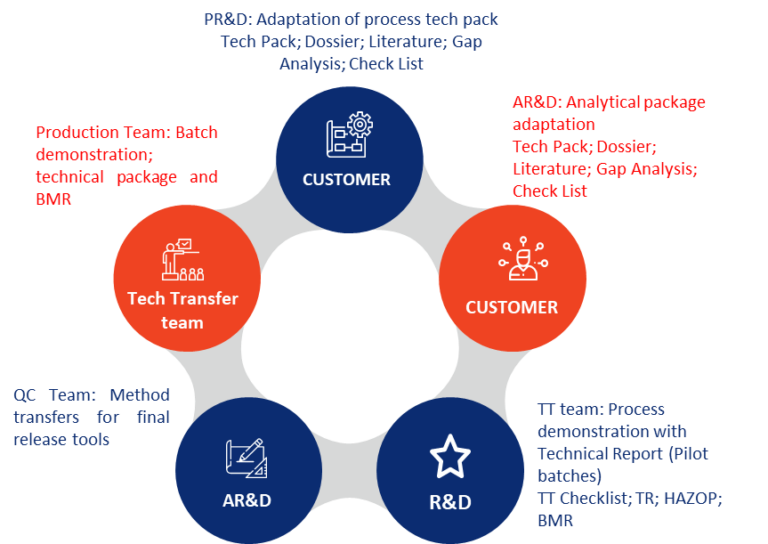
We employ a systematic, risk-based approach to technology transfer that ensures quality, compliance, and efficiency at every stage of the process. Our comprehensive approach includes:
Our Expertise
Our team consists of highly skilled scientists, engineers, and regulatory experts, all with decades of combined experience in drug substance development and technology transfer. We bring a deep understanding of process engineering, regulatory compliance, and industry best practices, ensuring that your technology transfer is both efficient and successful.
Our Services
The early phase is critical for establishing the foundation of your drug substance development. We support technology transfer from the lab to pilot scale, ensuring a smooth transition for process development and optimization.
As your product progresses to late-phase clinical trials, the focus shifts to scalability, process robustness, and compliance. Our late-phase technology transfer services ensure that your processes are optimized and ready for large-scale production.
As your product moves into the late-phase clinical trial stages, the emphasis transitions to ensuring that your manufacturing processes are both scalable and fully compliant with regulatory standards. Our technology transfer services provide the expertise needed to fine-tune and validate processes, ensuring seamless production scale-up while maintaining product quality and regulatory compliance.
As your product moves into commercialization, our focus is on ensuring large-scale production meets the required quality standards while maintaining cost-effectiveness and regulatory compliance.
Our extensive infrastructure, including cGMP pilot plants and kilo laboratories, ensures that processes can be smoothly transferred to large-scale manufacturing. We use software tools like Cheetah and Visimix to simulate and optimize large-scale processes, ensuring they meet quality and safety standards.
Why Choose Us?
At Aragen, we aim to streamline the transfer process, ensuring your drug substance development progresses smoothly while meeting global regulatory standards, maintaining product quality, and reducing risks.
Our services provide you with:
Our multidisciplinary team delivers phase-appropriate process R&D, ensuring robust, scalable, and safe development through route scouting, optimization, safety evaluation, and QbD principles.
Aragen ensures process safety and risk management across all phases, using advanced screening, risk assessments, and regulatory compliance for secure development.
Aragen provides end-to-end CMC support for IND applications, ensuring quality, compliance, and success through integrated process chemistry, manufacturing, and analytics.
