

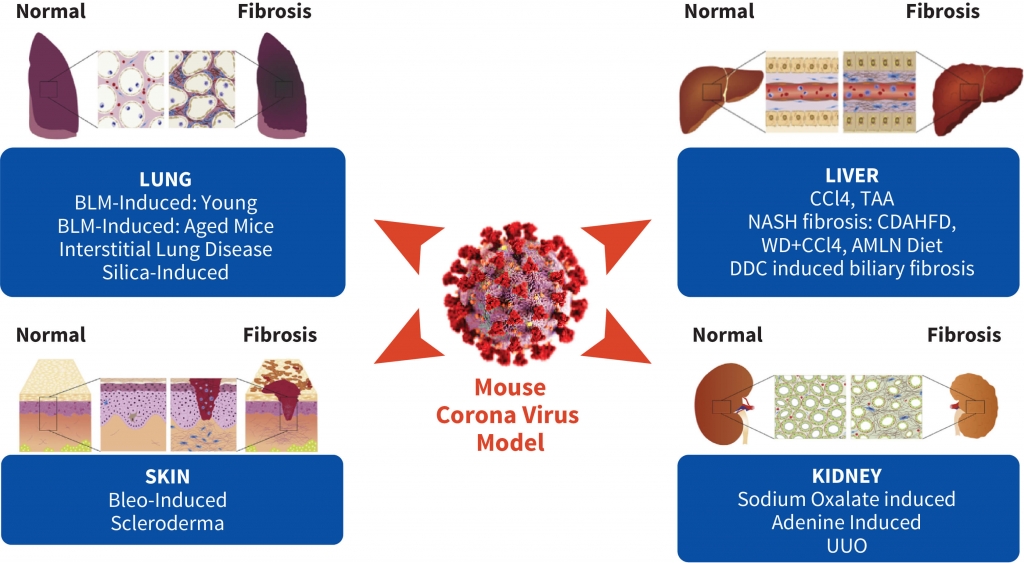
Fibrosis is an endpoint for multiple diseases and is driven by the excessive production and deposition of collagen and other extracellular matrix proteins in tissues and organs. The increase in tissue stiffness associated with fibrosis impacts the normal functioning of tissues such as the lung, kidney and liver.
Aragen has a portfolio of in vivo fibrosis models and world class expertise to support clients in the development of anti-fibrotic therapeutic or prophylactic candidates. In hundreds of studies, over the course of more than a decade, we have evaluated the efficacy of test compounds spanning several modalities (small molecules, biologics, RNA therapeutics, exosomes etc.) on a variety of assay platforms, ultimately resulting in INDs for our clients. Our diet-induced, chemical-induced and surgical models of fibrosis, coupled with an extensive evaluation of disease biomarkers, as well as cellular and molecular mechanisms, can help drive research efforts towards finding novel therapies.
MASH-Fibrosis Models to study Liver Fibrosis Aetiology
A complete replication of Metabolic dysfunction–associated steatohepatitis (MASH) arising from a variable interplay between environmental factors and genetic determinants is not possible. Nevertheless, preclinical animal models are essential to understand MASH pathophysiology and test antifibrotics. Among several mouse models of MASH, models based on overnutrition with adipose restriction/inflammation and metabolic complications, particularly insulin resistance, may be most useful to investigate critical etiopathogenic factors.
Some mice models that show inflammatory infiltrate and pattern of liver fibrosis observed in human MASH are good models for pharmacologic testing. On the other hand, mice that readily develop obesity with liver disease like human MASH serve as a good preclinical MASH model. These mice develop significant fibrosis due to specific genetic strains or mutations that cause consumption of diet enriched with fat, modest amounts of cholesterol, and/or simple sugars (“Western diet”). In addition, there are purely dietary models, such as high-fat/high-cholesterol, Western diet, and choline-deficient, amino acid-defined serve well as preclinical fibrosis models. MASH-related fibrosis in MASH models should show steatohepatitis and some focal bridging fibrosis, and etiopathogenesis. Scientists will work with clients and partners to discuss a minimum set of requirements that investigators and drug companies should consider optimizing pharmacologic therapies for MASH.
Case Studies:
Our scientists have created a customized, client-Specific study design in a mouse model for CDAHFD- induced MASH. The highlight of this model is as follows.
A customized, client-specific study design was developed in mice as part of the Western Diet + CCl4-induced model (Tsuchida et al., 2018 J Hepatol).
Human kidney fibrosis -relevant models that exhibit pathological tubulointerstitial fibrosis after induction of injury and with good reproducibility are the two most used mouse models for kidney fibrosis- folic acid (FA) induced nephropathy and unilateral ureteral obstruction (UUO). Aragen offers both these models for testing you antifibrotic drugs.
Pathogenesis of fibrosis is complex. The repetitive and constant injury “scarring” leads to a sustained and self-perpetuating activation of fibroblasts, leading to their trans differentiation into synthetic and highly contractile α–smooth muscle actin (αSMA). This leads to expression of myofibroblasts that deposit in extracellular matrix (ECM) causing stiffening and altering the normal lung architecture leading to decreased lung function. The matrisome of fibrotic ECM is a major process towards chronic disease progression. Multifunctional transforming growth factor–β1 (TGFβ1) is shown to be a key factor in various fibrotic diseases capable of triggering trans differentiation of fibroblasts into myofibroblasts . GFβ1 binding to the TGFβ1 receptor triggering downstream signaling by posttranslational modifications of cytoplasmic members of the SMAD family, which act as transcription factors in the cell nucleus, regulating the expression of common profibrotic genes, including ECM proteins. Plasminogen activator inhibitor-1 (PAI-1) is considered a therapeutic target option for fibrosis because it is a key signaling molecule in the TGFβ1 pathway. Also, in IPF, profibrotic interleukin-8 (IL-8) was found to be secreted by a special fibrogenic mesenchymal progenitor cell population with autocrine effects on proliferation and motility and paracrine effects on macrophage recruitment. Target-based antifibrotic drug discovery failed to go to clinics because many molecules are required to block multiple signaling pathways involved in fibrosis disease pathogenesis. On the contrary, pathway-unbiased phenotypic drug screening succeeded in discovery of first-in-class drugs. This success is attributed to the application of physiological relevant stimuli, and a readout close to the clinical end point observed in patient-derived primary cells. These developments resulted in rigorous effort for developing new and effective treatment strategies.
An exceedingly small number of efficacious compounds against IPF move to clinical trials, although several compounds show efficacy against pulmonary fibrosis in animal models. Hence relevant preclinical animal models are key to increase in success of preclinical IND-enabling process. We need to better identify, characterize, and select clinically useful targets in the animal models that CROs offer. Aragen scientists understand this dilemma and consequently offer most appropriate animal models. Our preclinical fibrosis services help in improving the identification and characterization of clinically relevant molecules or pathways responsible for progressive fibrotic diseases. We combine appropriate preclinical models, including models of Fibrosis, Lungs, Liver, Scleroderma, MASH-Fibrosis, Biliary fibrosis, and ex vivo (precision-cut lung slices) or in vitro models to assist your high-throughput drug discovery or validation of drug effects.
Case Studies:
Multiple studies were performed to achieve consistency in our methodology and the lung weight measurements as shown in the graphs below.
Bleomycin administration increased pro-inflammatory chemokines (MCP-1, MIP3a, MIP-2, IP-10) in group 2 (red) compared to no-bleomycin (control-blue) group.
Bleomycin induced skin fibrosis mouse and scleroderma models are extensively used to investigate the mechanisms of skin fibrosis and scleroderma to test potential therapeutics. Typically, Bleomycin is injected either daily or every other day, for 3–4 weeks to induce scleroderma in mice or by one time injection of bleomycin-poly (L-lactic acid) microspheres.
Aragen scientists have extensive experience in developing Bleomycin-induced scleroderma mice models. These basic protocols include:
