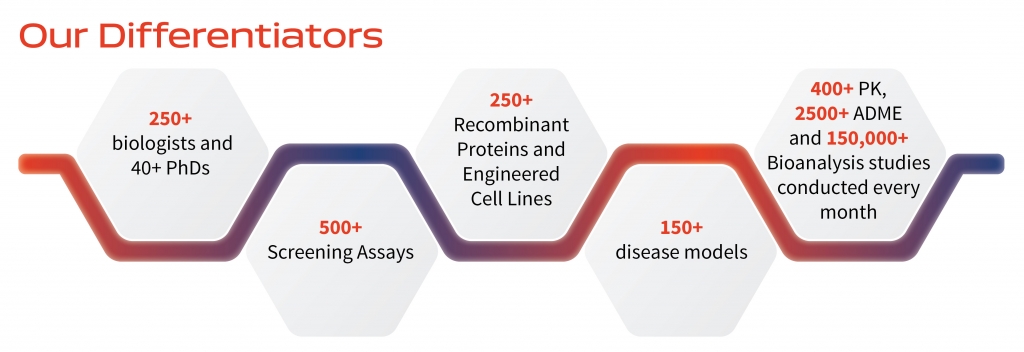
Aragen biologists are well-positioned to meet the diverse drug discovery needs of our global life sciences customers. We work in a consultative manner to build relationships of trust. Our biology experts become an extension of customer internal R&D teams, guiding them through the discovery continuum, from study design and execution to interpretation of data and recommendations on the path forward.
Aragen has an experienced team of in vivo pharmacologists with strong domain knowledge in various animal models of disease to obtain in vivo proof-of-concept across multiple therapeutic areas. We have validated models in Oncology, Inflammation, Autoimmune Disease, Pain, Fibrosis, Cardiovascular and Metabolic Diseases.
Our screening modalities include small molecules, large molecules and antibodies. We follow acute,chronic, mechanistic and pharmacodynamic models to assess target engagement, efficacy, PK/PD correlation and biomarker evaluation, in collaboration with our in vitro pharmacology and DMPK teams.
Our state-of-the-art vivarium and agile operational model enable quick allocation of appropriate space with technical staff to accelerate research programs.
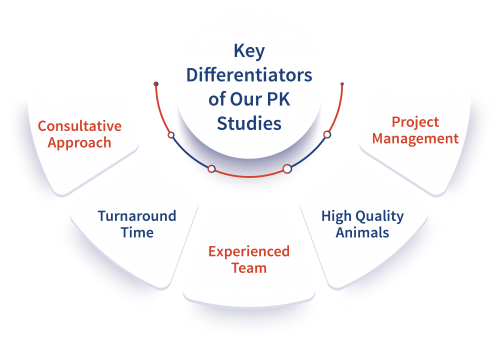
Consultative Approach
DMPK team provides consultative client support from study design, execution, data interpretation and suggestions of path forward.
Turn Around Time
Turn Around Time for our core quality DMPK solutions is less than 7 working days- one of the quickest among the CROs.
Experienced Team
DMPK team has experience in addressing challenging sponsor/ client requirements, working with big pharma, mid-sized pharma, virtual companies and academic institutions.
1300 PK
studies/month
12000 ADME
studies/month
300000
bioanalysis/month.
Aragen is a valued partner for gene to protein projects, offering specialized support from custom study design, drafting and implementation of workflows, to delivery of reagents. We have a solid track record of purifying 200+ functionally active recombinant proteins and generating 100+ cell lines as stable or transient expression systems for drug candidate screenings.
The integration of molecular biology, cell culture, protein purification and product analysis allow us to deliver complete packages of purified products, with associated functional and analytical data.
We also support structural and functional assays, target validation and throughput screens with extensive expertise in the production of recombinant proteins, monoclonal and recombinant antibodies, vaccine antigens and disease biomarkers as customized reagents.
Aragen in vitro pharmacology can facilitate lead identification and optimization of NCEs with end-to- end solutions in integrated drug discovery. These include target validation, optimization of biochemical, cellular and selectivity screens, the establishment of disease relevant secondary assays and the implementation of a biomarker strategy.
Our development of disease relevant assays spans multiple therapeutic areas, including Oncology, Immuno-oncology, Inflammation, Autoimmune Disorders, Fibrosis, Cardiovascular and Metabolic Diseases.
The identification, validation and screening of biomarkers is supported by diverse platforms such as qPCR, Luminex, flow cytometry, high-throughput Western blotting, high content imaging and LC-MS/MS.
We are also partner to major companies in targeted protein degradation (TPD), executing complete workflows across cell and protein sciences, assay development and screening of degraders.
