
Surface Plasmon Resonance (SPR) is a cutting-edge, label-free technology used to study biomolecular interactions in real-time. It detects changes in the refractive index near a sensor surface when molecules bind to an immobilized partner, allowing researchers to analyze the kinetics and affinity of interactions between biomolecules, from small ligands to large complexes. SPR is particularly well-suited to detect binding interactions at any site on a protein, making it an invaluable tool in the development of heterobifunctional degraders, such as Proteolysis Targeting Chimeras (PROTACs), for therapeutic use.
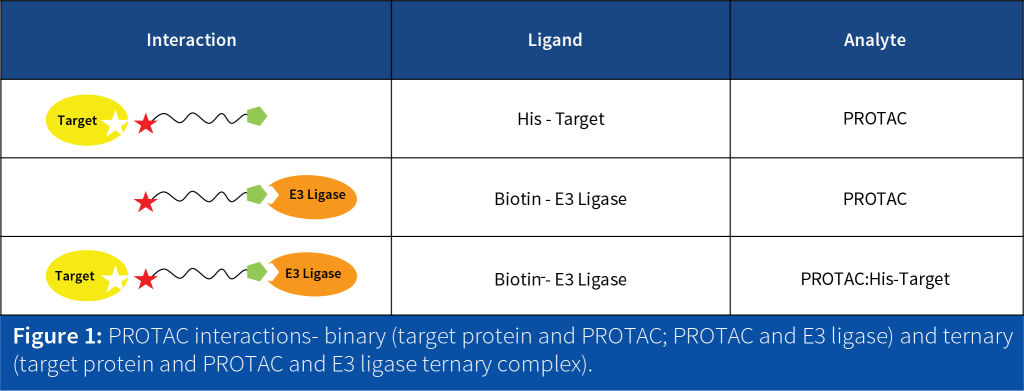
PROTACs are bifunctional molecules designed to induce the targeted protein degradation by binding to both a target protein and an E3 ligase, forming a ternary complex (Figure 1). The ternary complex promotes the ubiquitination and subsequent degradation of the target protein by the proteasome.
Analyzing complex molecular structures, such as ternary complexes, is inherently difficult due to the intricate interactions between multiple binding partners, which complicates the study of their kinetics and binding affinity. Conventional techniques like Fluorescence Polarization (FP), Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) and Amplified Luminescent Proximity Homogeneous Assay (AlphaLISA) can study ternary complex formation but cannot provide label-free kinetic analysis for both binary and ternary complexes. Isothermal Titration Calorimetry (ITC) provides thermodynamic data but is limited by low throughput, high sample requirements, and lacks kinetic insights.
SPR overcomes these challenges by enabling label-free, real-time measurement of binding kinetics and affinity for both binary and ternary complexes, making it ideal tool for optimizing PROTAC kinetics and augmenting targeted protein degradation.
At Aragen, we utilized SPR to characterize the stability and efficacy of a PROTAC-target-E3 ligase ternary complex. The experimental strategy is outlined below:

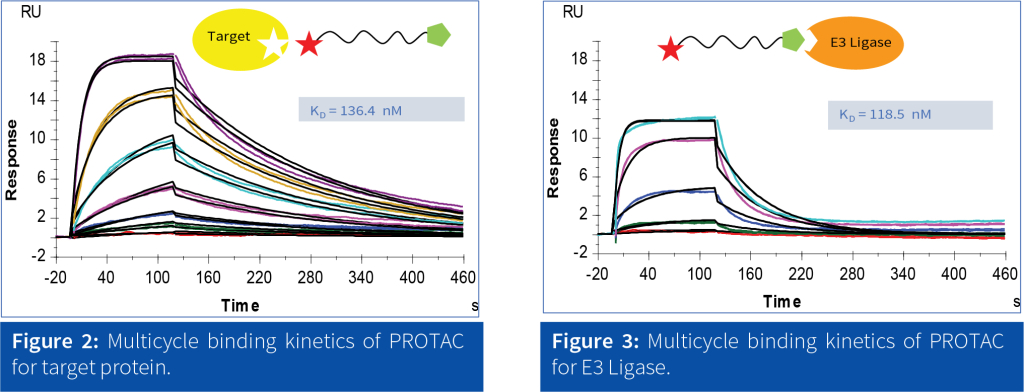
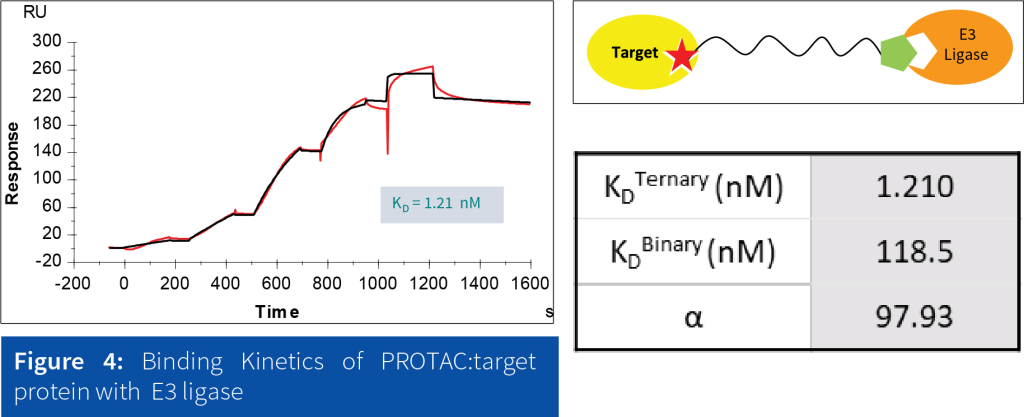
SPR has proven to be an effective technique for studying the binding affinity of PROTAC interactions and optimizing their eficiency in targeted protein degradation. It offers:
These insights underscore the applicability of SPR technology in optimizing PROTAC design, ensuring better stability, selectivity, and efficiency in targeted protein degradation.
With nearly a decade of SPR expertise, we accelerate drug development by providing trusted insights that optimize drug candidate profiling and efficacy. We offer:
