
The testing and assessment of Extractable and Leachable substances in finished drugs and their packaging material is extremely important to mitigate the patient safety risk associated with harmful product contact
materials. Extractables and leachables can lead to potential contaminants in the final product and may impact product efficacy, color, taste, smell, and its pH. Other effects may involve inactivation of the active ingredient and increase in the carcinogenic risk associated with the drug.
Extractable and Leachable studies are therefore a crucial component for the safe release of a drug into humans, and help identify impurities that migrate into the final product from contact surfaces encountered during drug manufacture and storage.
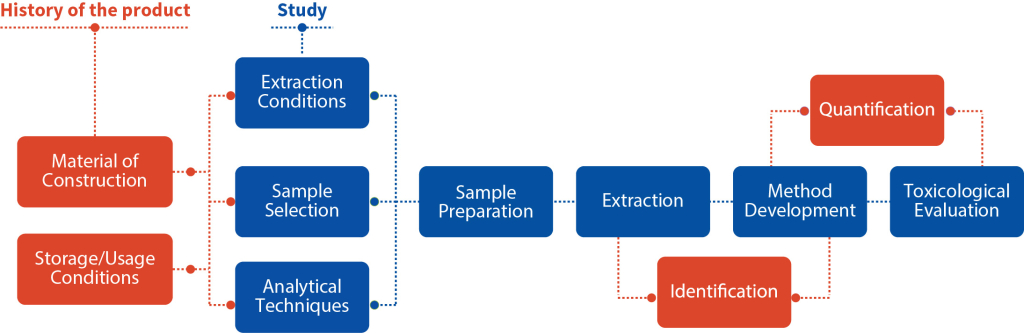
Conducting Extractables and Leachables studies is a complex, multi-step process. At Aragen, we carry out a comprehensive Extractable and Leachable testing program in accordance with global regulatory
recommendations and guidelines. These studies can either follow standard procedures or can be tailored to meet specific customer requirements. Supported by a world-class infrastructure, our analytical experts assess your needs, develop protocols and then drive method development, method validation and stability testing, enabling you for successful and safe product launch.
Our study design aims at examining every aspect of the drug product and packaging in accordance with regulatory requirements. These include:

| Analytical Technique | Extractable/Leachable | Example |
|---|---|---|
| LCMS | Non-volatile organic compounds | Nitrosamines, Antioxidants, Oligomers |
| GCMS (Direct injection) | Semi-volatile organic compound | PAHs , Plasticizers, Preservatives, Siloxanes |
| GCMS (Headspace) | Volatile organic compounds | Processing solvents, Adhesives, Siloxanes |
| ICP-MS | Non-volatile organic compounds | Arsenic, Lead, Chromium |
| Critical examples from the past – pharmaceutical packaging area (MDI) | Polyaromatic hydrocarbons (PAHs), mercapto-benzothiazole and N-nitrosamines; (prefilled syringes) tungsten case & photoinitiators |
| Critical examples from the past – food packaging area | ITX and other printing compounds and N-Nitrosamines |
| Scientific reasons | Additives and process chemicals are small molecules, some pharmaceutical formulations are quite good solvents for such extractables |
| To avoid surprises | Degradation products of polymeric matrix or additives. Unknown compounds from manufacturing of the packaging (“NIAS”) and contaminants. Compounds migrating from printing and adhesives into the drug products |
| Regulatory Guidelines | USP, EP, FDA, EMEA |
