
Glioblastoma multiforme (GBM) is a highly aggressive brain cancer and poses a significant challenge in oncology due to its aggressive nature, marked by rapid proliferation, infiltration, and treatment resistance. To address this, Aragen Bioscience offers the orthotopic brain tumor model—an indispensable tool for advancing GBM research. Unlike traditional models, the orthotopic model closely simulates human physiology, providing a natural environment for studying the disease, testing treatments, and understanding drug delivery.
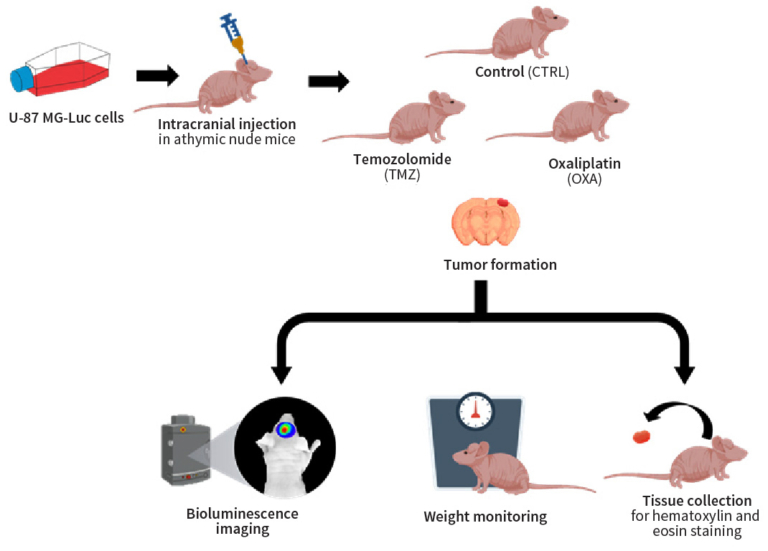
In this study, we established a luciferase-based orthotopic human GMB model by intracranial injection of a luciferase-tagged human glioblastoma cell line, U-87 MG-Luc, in athymic nude mice. The model is designed to precisely monitor tumor growth and therapy response through non-invasive bioluminescence imaging (BLI), which is crucial for the preclinical evaluation of anti-GMB therapeutics and a pivotal step preceding clinical trials in patients.
Female athymic nude mice were intracranially injected with U-87 MG-Luc cells to develop gliomas. Thereafter, the glioma-bearing mice were randomly distributed into control and treatment groups (temozolomide, TMZ, and oxaliplatin, OXA).
We assessed tumor progression and treatment efficacy via BLI over 35 days, concurrently monitoring body weight changes for adverse effects.
Median survival time was determined through Kaplan-Meier plot analysis. Histological examination of glioma tissues excised from control and treatment groups was performed by hematoxylin and eosin (H&E) staining. Additionally, blood cell count analysis was conducted to evaluate myelo-suppression in both treatment and control groups.
Aragen Bioscience team employed the orthotopic brain tumor model to simulate the GMB microenvironment and monitored tumor growth in real time using non-invasive BLI. The plot of bioluminescent signal emitted from the tumor over time revealed that both TMZ and OXA treatment significantly reduced the tumor burden compared to the no treatment group (Figure 1A and 1B). Noteworthy, OXA treatment led to significant weight loss, indicating a potential adverse effect (Figure 1C).
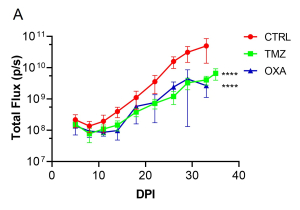
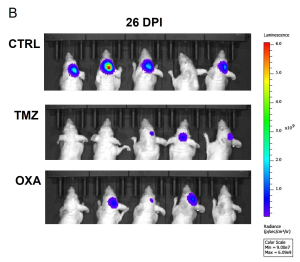
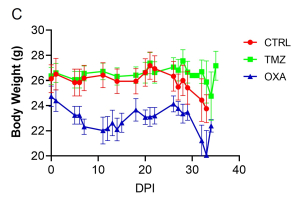
Figure 1: Tumor burden and body weight over time.
(A) Average bioluminescence imaging (BLI) signal per experimental group [control: CTRL and treatments, TMZ or OXA] over 35 days. ****p<0.0001 by 2-way ANOVA, compared to CTRL. (B) Bioluminescence images at 26 days post implantation (DPI). (C) Average body weight of each experimental group over 35 days.
The Kaplan-Meier survival curve demonstrated that TMZ had a trend to improve survival in comparison to CTRL group (Figure 2A). Furthermore, TMZ had minimal immunosuppressive effects compared to OXA as revealed by the blood count analysis (Figure 2B). Our findings from H&E staining (Figure 3) were consistent with BLI outcomes, elucidating the chemotherapeutic activity of both TMZ and OXA against glioma. The results from our study corroborated with previous research (Melodie Davy et al., cancers, 2023; Liliana Soroceanu, etal. Neuro-Oncology Advances, 2022).
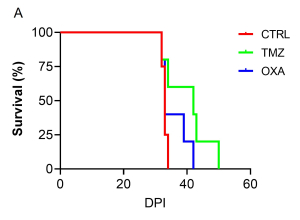
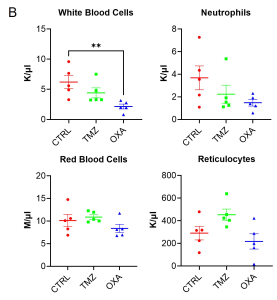
Figure 2 : Survival plot and whole blood CBC analysis.
(A) Kaplan-Meier survival curve of glioma-bearing mice with no treatment (CTRL) or treated with TMZ or OXA. (B) Complete blood cell count analysis from whole blood on 34 DPI. **P<0.01 by 1-way ANOVA followed by Dunnett’s post hoc test. K/µL = Thousand/µL; M/µL = Million/µl.
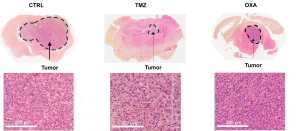
Figure 3: Brain Glioma H&E Staining
H&E-stained whole brain slices (at low magnification) and tumor cells (at high magnification) from untreated (CTRL) or TMZ- or OXA-treated glioma-bearing mice on 32 DPI.
Development of orthotopic glioma models using cell lines requires accurate localization of the injection site and depth within the brain during intracranial injection procedure. Even slight deviations in targeting can lead to inaccurate results or unintended tissue damage.
Aragen Bioscience’s scientists have successfully developed and validated luciferase-based orthotopic human GMB model, using U-87 MG-Luc cells in athymic nude mice. The model enables precise monitoring of tumor growth and therapy response through non-invasive bioluminescence imaging and facilitates a comprehensive preclinical evaluation of potential anti-GMB therapeutics.
Trust us to propel your GBM research forward with our cutting-edge orthotopic model services. With our team’s expertise in aseptic surgery, meticulous planning, careful monitoring of both the injection process and post-injection outcomes, besides execution of subsequent experimental procedures, we offer a controlled and pertinent platform for understanding glioblastoma biology and assessing therapies, advancing towards an improved quality of life for GBM patients.
We gratefully acknowledge the Charles River Laboratories for their invaluable support by providing the animals used in this study.
