
Pharmacokinetic studies of lung targeted drugs are generally limited to measurement of systemic plasma concentrations that gives no direct information on lung target site concentrations. The estimation of lung target site concentration of the inhaled drug is important as it directly provides the efficiency of the mode of delivery of the drug. The mode of delivery is considered more efficient if the local concentration of the drug achieved is higher in the lungs, compared to the plasma concentration.
The inhalation mode of administration is preferred for the treatment of pulmonary complications because it delivers the medication directly to the target organ, improving pulmonary specificity and reducing systemic side effects. A high therapeutic ratio for the inhaled route of administration is achieved by employing efficient devices and delivering doses which achieve a high local concentration in the lung and relatively low levels of systemic
absorption.
Here in this article, we demonstrate a study performed by the expert DMPK scientists of Aragen to measure the mean plasma and lung concentration of the model drug Salbutamol (Asthalin respirator solution) administered via inhalation mode (nose only exposure) in CD-1 male mice, using the inExpose instrument. inExpose is a product of SCIREQ, designed to operate under a standard fume hood and distinguishes itself by its compact size and high level of integration. Its modularity and integration permit both nose-only and whole-body exposure of rodents.
Salbutamol administered via inhalation mode (nose only exposure) in CD-1 male mice, using the inExpose instrument.
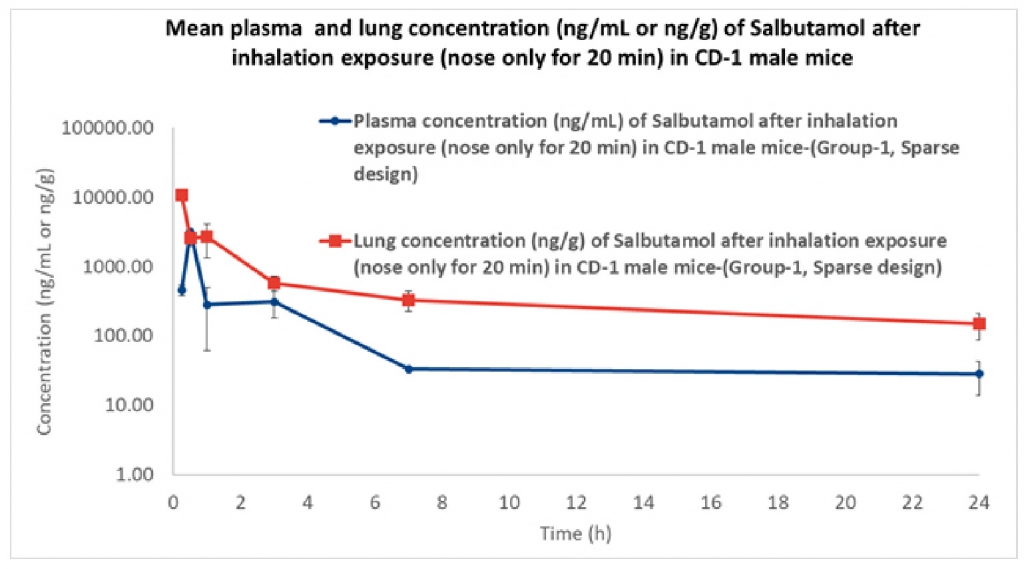
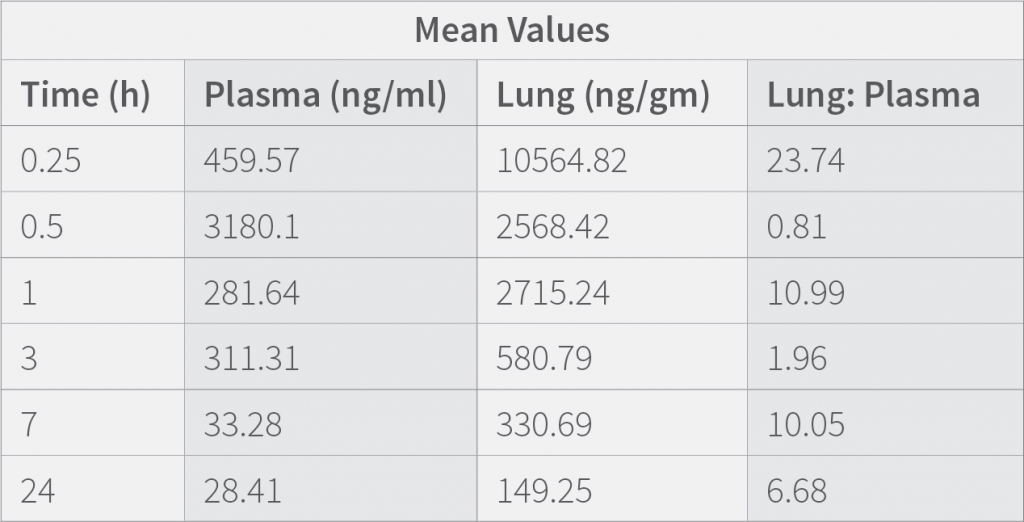
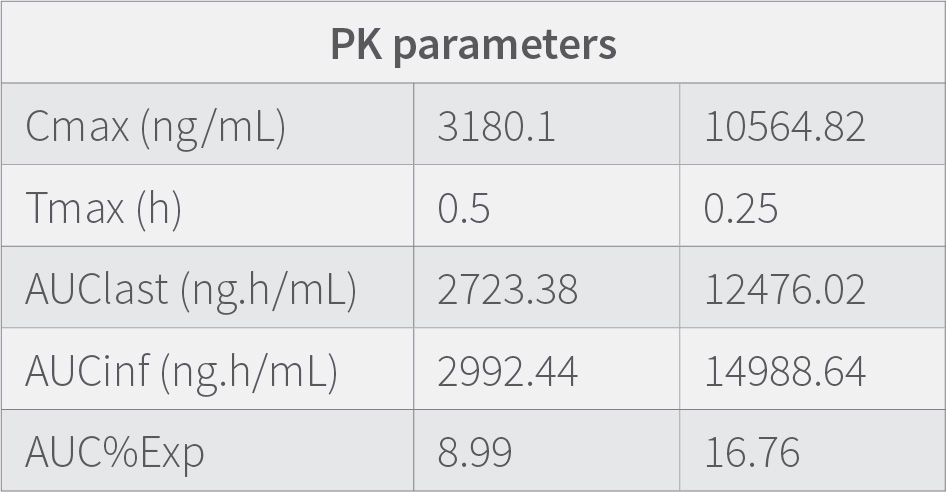
Results demonstrates that the concentration of the drug in the plasma at 0.25 h is low compared to when measured at 0.5 h. showing that the medication requires time to get absorbed in the plasma, The concentration of the drug in lungs is highest at 0.25 h and then decreases suddenly from 0.25 h to 0.5h and then gradually decrease till 24h. Results also indicate that the therapeutic ratio of the drug (Lung to plasma ratio) is high initially (0.25 h) and decreases as the time lapses. At any given time, the lung concentrations are higher than systemic circulation.
Aragen successfully conducted the drug administration studies in CD-1 male mice through inhaled route of administration by nose only exposure employing inExpose instrument. Aragen has capabilities to conduct similar studies for range of molecules and establish high quality pharmacokinetics data in competitive timelines.
Aragen Life Sciences is a leading R&D and manufacturing solutions provider for the life sciences industries worldwide. It offers end-to-end integrated or standalone solutions to advance small and large molecule programs from concept to commercialization. Established in 2001, the Company operates through a global network of six sites with a team of 3800+ employees and 450+ PhDs. Its expertise and experience have enabled over 450 customers (including 7 of the top 10 pharma companies globally) in advancing their research programs from early discovery through development and commercialization.
Aragen life sciences is a leading service provider for conducting drug metabolism and pharmacokinetics (DMPK) studies of new drug candidates. We conduct comprehensive and cost-effective DMPK studies to evaluate and optimize the drug-like properties of range of novel molecules (small and large) targeting various diseases including pulmonary complications. We incorporate customized approaches and advanced technologies such as high-throughput screening to provide decision enabling, high quality data with rapid turnaround time.
