At Aragen, we adopt a comprehensive developability approach, commencing from the conceptualization stage of candidate compounds. Early development roadmaps are important for two reasons: first, to understand the physicochemical behavior of a molecule to better predict its success on the path to commercialization; and second, to understand and assess challenges that a product may encounter during scaling-up or commercialization.
The CMC developability assessment in different organizations is employed through standard protocols developed over the years based on trial and errors of different methods. Mostly, the assessment methods employed include both in silico analysis as well as in vitro assays to access the solubility, integrity, thermostability, potency, quality, and other essential characteristics.
Each therapeutic protein is unique and biological systems are not always predictable. Developability assessment method employed for a particular biosimilar may not necessarily work for another protein due to the number of domains, secondary structure, molecular weight, or conjugation with different ligands. Strategizing the developability assessment of a particular biologic demands a unique standardization method that will include all the parameters like aggregation, charge heterogeneity, glycosylation, potential PKPD profile etc. The growing interest in increasingly complex compounds like bispecific monoclonal antibodies and Fc fusion proteins necessitates this evaluation, rendering timely screening even more integral.
To assess the developability of therapeutic biologics, Aragen uses both large and small-scale, rapid, and predictive approaches. To de-risk the clinical manufacturing of a range of biologics, Aragen creates unique scaling up methodologies for each molecule, considering its ability to combat a particular disease.
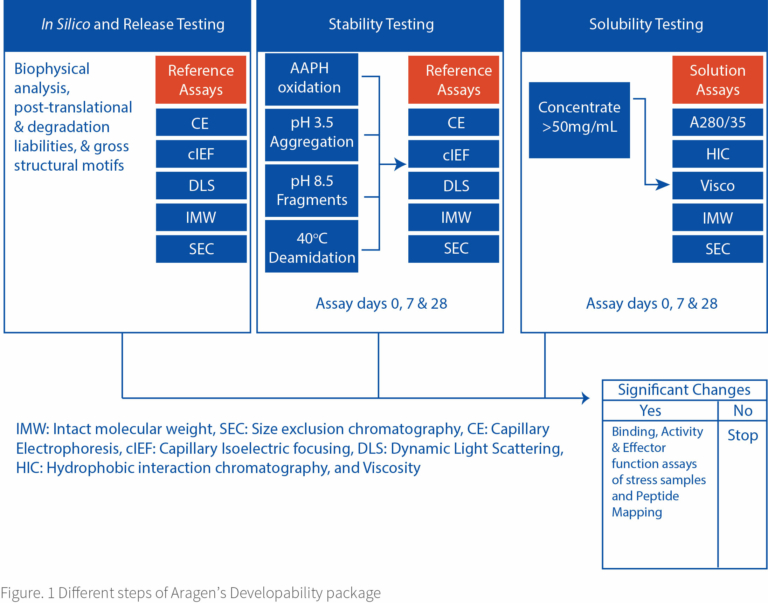
At Aragen, we start with in silico and release testing to access the required biophysical and chemical properties of the hit molecules. We thoroughly understand the secondary and tertiary structures and account for all the structural motifs of the biologic. Understanding posttranslational and degradation liabilities enables the engineering of high-producing cell lines expressing the biologic. We further screen the candidates based on their behavior against different pH levels and temperature ranges to estimate their melting temperatures and aggregation profiles. In stability testing, we stress out the molecules for oxidation or extreme pH and temperature ranges and study the resilience of different candidates.
Solubility assessment in different solvent systems and in varying concentration ranges identifies precipitation behavior and establishes dosing amounts. Stability and solubility studies are performed on different time periods, which is necessary to identify the robust candidates and enable the design of the optimal up-scaling and manufacturing protocols. We find possible drug candidates by analyzing the results of numerous assays, and in situations where many candidates exhibit promising behavior, we use methods such as peptide mapping, binding to various conjugate compounds, and activity and effector function tests to further screen them. Figure. 1 represents the brief graphical representation of the flow we adopt for developability assessment.
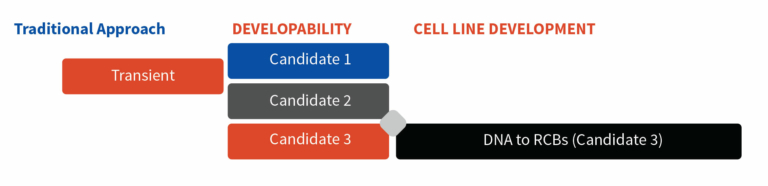
Conventional approaches screen potential candidates based on the conclusions derived from the developability package and then commence cell line development only for the candidate who has been finalized. Conventional methods are time-consuming, as the cell line development begins only after the developability assessment.
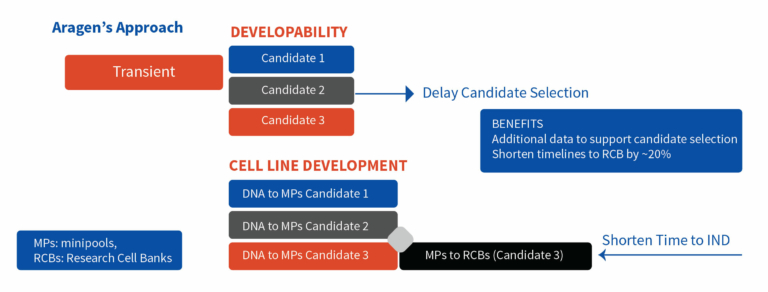
At Aragen, we start cell line development for all the hit molecules that are being evaluated for developability. All the cell line development processes go through the minipool stage, and at that point, we identify the best candidate based on both the developability assessment and the quality of the minipools. RCBs are developed only for the selected candidate, saving almost 20% of the time. Figure 2 demonstrates the difference between the conventional approach and Aragen’s approach to move from the transient material to final RCB.
To know more about Aragen’s developability assessment capabilities, please write to bd@aragen.com and our subject expert will be happy to connect with you.