
In drug discovery projects where novel drug candidates are being evaluated against target molecules (proteins) it is crucial to first identify the correct target and secondly to quantify the rate of formation of drug-protein complexes over time. It is also essential to know the saturation concentration of the drug corresponding to the target protein, in order to determine the suitable and efficacious doses of the drug molecule. Standard approaches to detect and quantify the protein or protein-drug complexes are experimentally challenging and require advanced instrumentation, high-quality reagents, and experienced manpower. Recent approaches for drug-protein quantification involves techniques of molecular biology and analytical chemistry wherein expression of the target protein either in the endogenous cell line (naturally expressing) or in engineered cell line is required to understand a holistic mechanism of drug interaction with its target molecule/protein.
Once the protein-drug complexes are pulled down via chromatin immunoprecipitation, accurate detection and quantification of the complexes is done using traditional approaches like ELISA or the advanced techniques like Mass Spectrometry.
ELISA is a popular protein detection immunoassay. ELISAs have dominated protein detection because of their high throughput, sensitivity, and selectivity. Furthermore, ELISA is less complicated and less expensive than mass spectrometry. However, the former is the least bet when it comes to new proteins or Novel Biological Entities. In the experiments involving quantification of drug-protein complexes, 100% accuracy is required, and potential protein contaminants needs to be identified because of the serious deleterious effect of protein-drug complexes. ELISA cannot detect all the contaminants accurately and sometimes can also give false positive. In addition, developing an ELISA assay is time and reagent consuming process.
Mass spectrometry can measure a variety of characteristics that ELISA cannot. In addition to evaluating the product impurity profile, mass spectrometry may discover low abundance protein-drug complexes. Mass spectrometry is also used to monitor the clearance of individual host cell proteins (HCPs) that have known effects on drug efficacy or patient safety. The biggest strength of Mass spectrometry is specificity and accuracy of the data generated which can be relied upon 100%. Moreover, long term cost of running samples in mass spec becomes cheap once the method has been developed and validated.
Here in this article, we report a study conducted by the Aragen DMPK group. The objective of the study was to quantify the drug-protein complex through mass spectroscopy and to determine accurate Kinact and Ki values. The challenge was to titrate the correct concentrations of the target protein and drug ratio to achieve an optimized and unbiased assay.
The client approached Aragen to detect the interaction between a protein having molecular weight (MW) of >27000 Daltons and novel drug candidate having MW of ~430. To accurately detect the interaction between the target protein and the drug candidate mass spectroscopy technique was implemented. Furthermore, extensive experimentation at various concentrations of the drug molecules within a time course were conducted to calculate the saturation concentration of the drug molecules at which their binding potential to the target protein is highest.
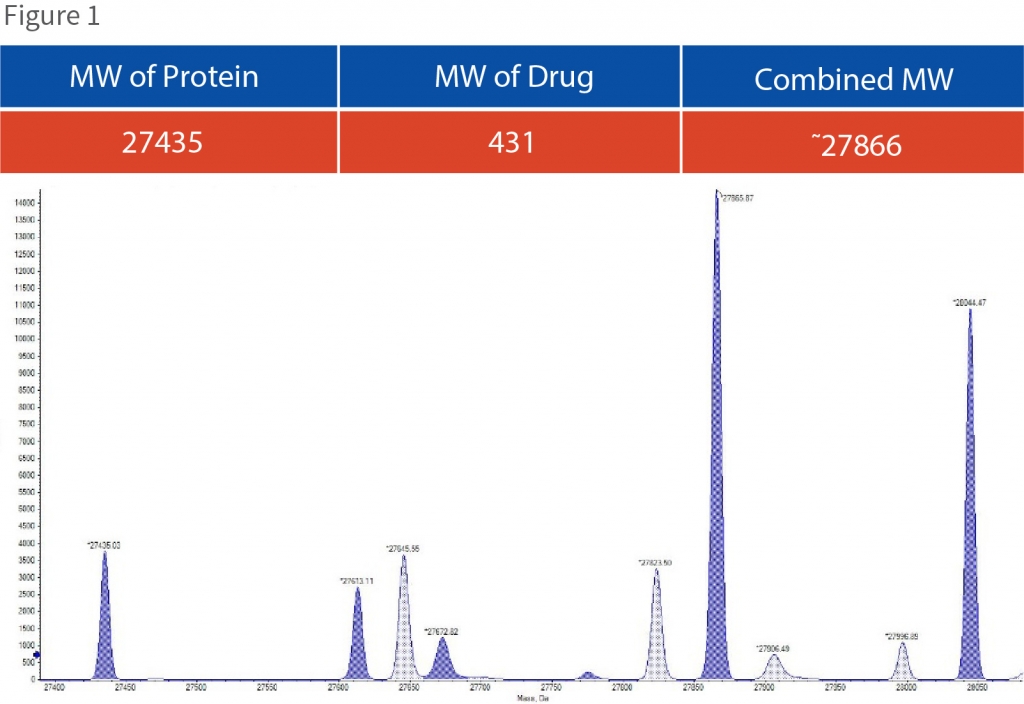
We conducted mass spectrometric–based assay to determine covalent binding of Novel drug candidate to the target protein. Figure 1 demonstrates the results indicating the presence of the target protein, drug candidate and the kinetics of complex formation. The graph also shows various other peaks confirming the impurities in the sample along with the pure functional protein.
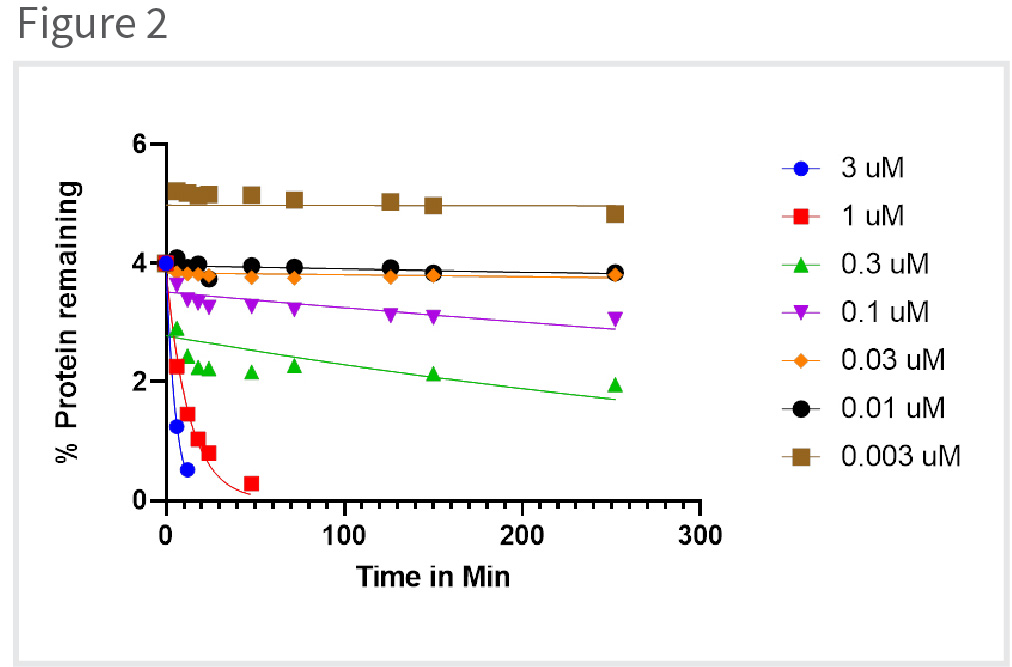
To determine the Kinact and Ki values, different experiments were performed at various concentrations of the drug candidate while keeping the protein concentration constant (Figure 2). The first objective of the experiment was to determine the concentration of the free/unbound protein in the presence of the drug over the course of time. Different concentrations of the drug candidate that were considered for the analysis were 0.003, 0.01, 0.03, 0.1, 0.3, 1 and 3 uM.
When the concentration of the drug is less than the unbound protein is high in the initiation of the experiment, and it decreases with time. Increasing the concentration of the drug results in the decreases in the initial concentration of the unbound protein and it also decreases as the time progresses.
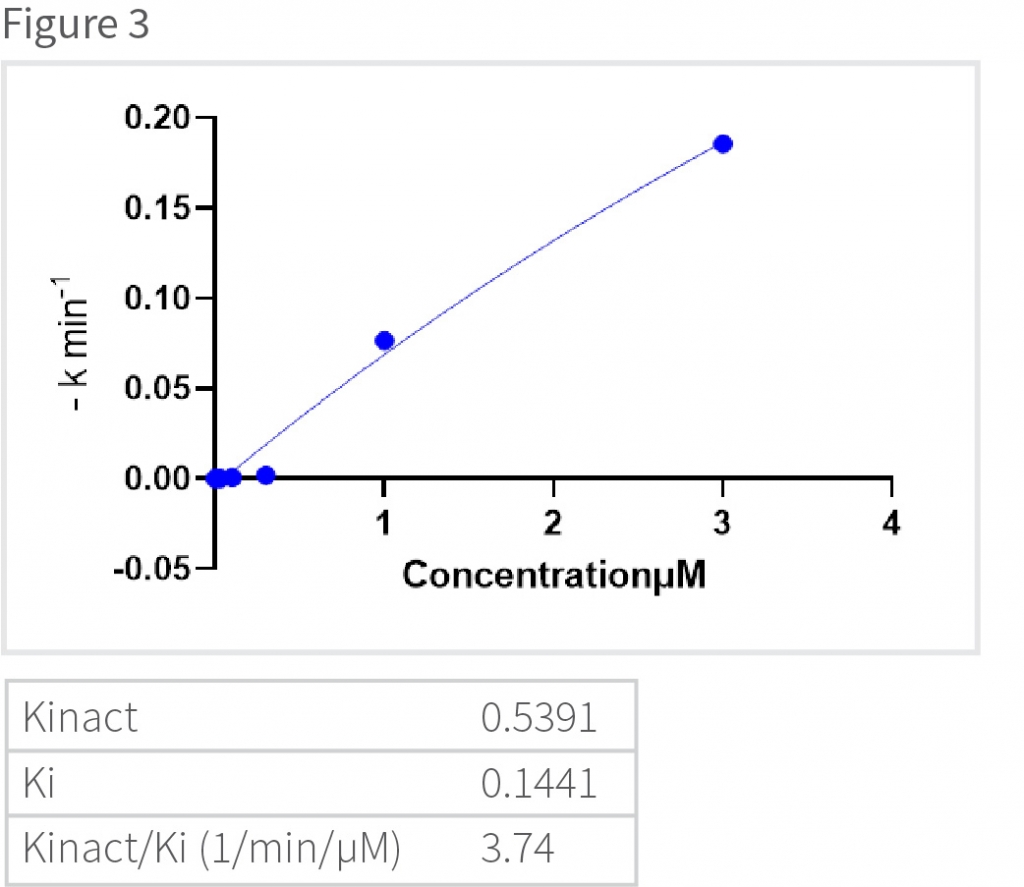
The slope was calculated for all the concentrations of the drugs and plotted against the different concentrations of the protein to calculate the Kinact (min-1) and Ki (µM) values (Figure 3). Results show that for the model protein the Kinact is 0.5391 and the Ki value is 0.1441.
Aragen successfully developed a Mass Spectroscopy based assay for the detection of the target protein and a novel drug molecule. The assay also established the successful protein-drug complex as demonstrated by the MS peaks. Target engagement assays were performed and the Kinact and Ki values were successfully established.
Our expert scientific personnel established accurate and reproducible data for drug-protein complexes and delivered to the client. The data was thoroughly tested and validated, and the client exhibited full confidence at the protocol established. It was also demonstrated that the protocol generated is applicable to other drug-protein complexes in the field of Global Proteomics, Phospho-proteomics, and Pharmacogenomics.
Aragen Life Sciences is a leading R&D and manufacturing solutions provider for the life sciences industries worldwide. It offers end-to-end integrated or standalone solutions to advance small and large molecule programs from concept to commercialization. Established in 2001, the Company operates through a global network of six sites with a team of 3800+ employees and 470+ PhDs. Its expertise and experience have enabled over 450 customers (including 6 of the top 10 pharma companies globally) in advancing their research programs from early discovery through development and commercialization.
Aragen life sciences is a leading service provider for conducting drug metabolism and pharmacokinetics (DMPK) studies of new drug candidates. We conduct comprehensive, mechanistic, very competitive turn around time and cost-effective DMPK studies to evaluate and optimize the drug-like properties of range of novel molecules (small and large) targeting various diseases. We also possess expertise in conducting targeted proteomics and protein quantification services.
