
Avoiding cryogenic conditions, reducing solvent consumption by 50% and IPC conversion > 96%
Butyl Lithium chemistry is extremely difficult to execute for production chemist’s, which requires cryogenic reactors and pose a very high risk of run away. In batch reactors Butyl Lithium reactions are done at cryogenic conditions typically at temperatures below -50 °C to avoid undesired side reaction. Also, large volume of solvent is required to control the heat generated during the reaction.
Adopting flow chemistry provides an efficient, safe, and economical platform for handling reagents like Butyl-Lithium due to the inherently high heat and mass transfer rates in flow owing to high surface to volume ratio. In flow reactors, since heat transfer rates are very high, these cryogenic conditions can be avoided, and solvent quantity can be reduced to up to 50%.
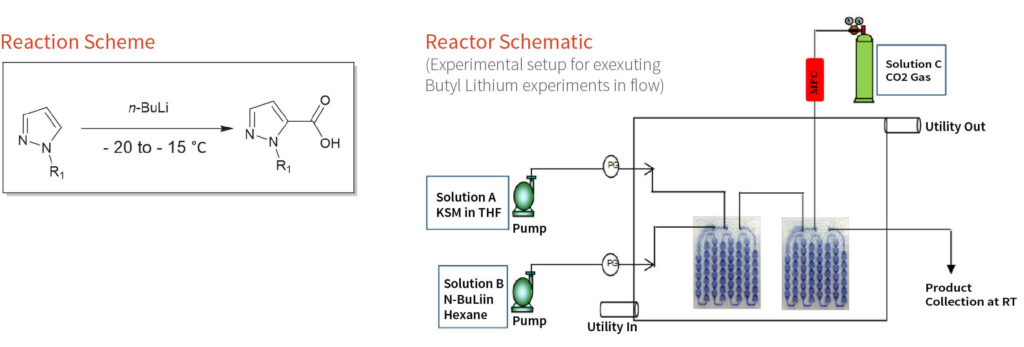
In this case study, we present one of the projects executed by our researchers, which involved conducting lithiation on a key starting material (KSM) and subsequently attaching a carboxyl group to it. Batch reactions posed challenges in terms of execution, energy expenditure, and the generation of significant impurities. Therefore, a flow chemistry setup was adopted, considering its advantages over a batch setup. Aragen successfully conducted flow chemistry experiments involving highly hazardous Butyl Lithium reactions and achieved savings in utility costs and solvent consumption of approximately 70% and 50%, respectively. Additionally, flow reactions are greener and easier to scale up.
Executing the butyl lithium reaction of a KSM in batch and identifying the preliminary optimal parameters for executing the reaction and recognizing the challenges inherent in a batch setup. The key objective of the project was to optimize the parameters in a flow setup, that would enable the reaction to take place at room temperature (RT), reduce utility costs and solvent usage, and minimize impurity formation during the reaction.
To determine the best parameters for conducting the reaction in a flow setup, enabling it to occur at room temperature while minimizing the formation of impurities. These parameters should also align with the principles of green chemistry.
The client is a pharmaceutical research company based in Denmark, with subsidiaries located worldwide. Their services include research and development, the large-scale synthesis of key intermediaries, active pharmaceutical ingredients (API), and the formulation of various products in a regulated manner.
A series of batch experiments were conducted to determine the best parameters for product formation. Initial flow experiments were conducted with the same parameters identified in the batch setup. Various experiments were carried out to pinpoint the parameters that would yield minimal impurities, reduced utility costs and solvent usage, and smooth execution at room temperature. The flow setup involved the use of two HPLC pumps to pass the KSM in solvent and n-butyl lithium in hexane. The flow rates were adjusted to maintain the reaction stoichiometry. Additionally, the reaction mixture was passed through one microreactor where the lithiation of the KSM occurred, while in the second microreactor, CO2 was introduced to react with the intermediate product, facilitating the attachment of the carboxyl group.
Key achievements from flow trials of n-Butyllithium reactions
• IPC conversion of more than 96% achieved
• All known and unknown impurities are well controlled and below 0.1%
• Temperature of reaction +20 °C
• Overall residence time of reaction less than 1 min
• Operationally smooth and safe
Key advantages from flow trials
• High process control strategy compared to traditional batch reactors
• Low handling volumes
• Easy and seamless scale-up
• Huge utility savings
• Greener process with reduced solvent quantity
