 Back to News
Back to NewsElectron mobility with photoredox catalysis
March 16, 2022
Dr Somesh Sharma, PhD, senior vice president and head of discovery chemistry solutions at Aragen Life Sciences, discusses how photochemistry offers an alternative to conventional processes for functional transformation in organic synthesis
Nature has always inspired scientists over the years. Now, there is a big buzz about sustainability and carbon zero technological platforms to build a safer and healthier environment for future generations. Chemists generally employ conventional processes (thermal or catalytic activation) for any kind of functional transformation in organic synthesis.
There is an urgent need to make chemical conversions greener, safer and environment-friendly. Photochemistry and especially photocatalysis provide a suitable alternative to traditional approaches: a disruptive and transformative platform learned from nature of harnessing light to thermal energy, for instance, vitamin D and photosynthesis processes.
The implementation of any new technology hinges on its versatility, modularity, scalability, safety and sustainability. Early perceptions on photochemistry, however, were to overlook and avoid it because of the intractable challenges of reproducibility, robustness and scalability – an attenuation effect of photon transport.
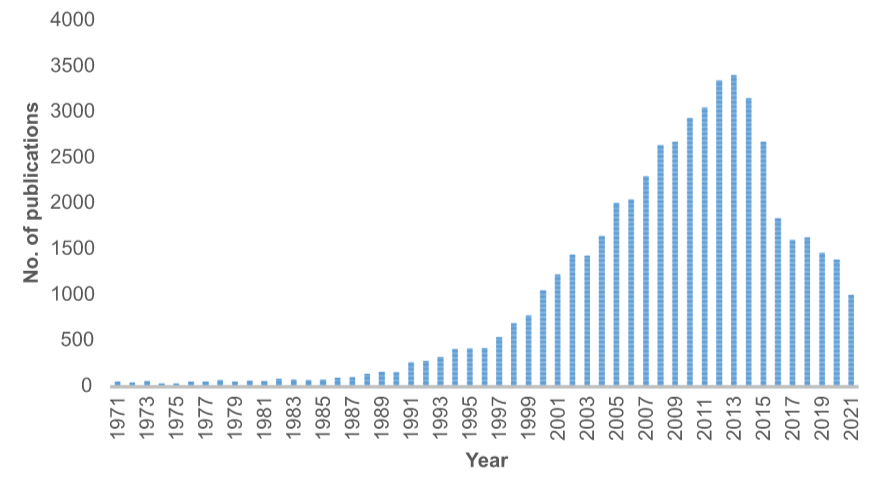
Figure 1 – Publication counts on photoredox catalysis
A huge surge has been witnessed in the last few decades (Figure 1), along with increased knowledge of organic and organometallic species, and their distinct properties in converting light to chemical energy for new chemical bond integrations. Generally, a photoredox catalysis (PC) process is established, wherein a single electron transfer (SET) between the excited state of a photocatalyst and organic substrate gives rise to highly reactive species, yielding unique products.
Selection parameters
To make any photochemical process viable its fundamental concerns – the photon source, the reaction medium and the type of vessel – need to be addressed. UV-induced photochemistry is well reported in literature via [2+2] cycloadditions and Norrish type reactions arising from homolytic bond cleavage.
These forms of radiation have limitations on selectivity and tolerability owing to the high associated energy and broad wavelength range. The deployment of appropriate wavelength filters, such as perfluoroalkoxyalkanes (PFAs), perfluoroethylenepropylen (FEP), quartz, pyrex and corex, and light sources with a narrow spectral range, is therefore preferred.
LED lights have circumvented some of the issues but are plagued with the inherent problem of low intensity and lifetime stability. Efforts are being made to develop high performance LEDs stemming from sapphire technology, so as to produce highly efficient lights with narrow spectral ranges.
Categories of PCs
There is always a quest to conduct photochemical reactions in the visible region for milder reaction conditions, eco-friendliness, and reliability. Interestingly, the issue is addressed through the usage of PCs, their propensity to harness light energy, getting excited to reactive species (Figure 2) and manoeuvering electron mobility by the redox potential of reaction media to construct new chemical bonds via heat transfer, isomerisation, degradation, photosensitisation, reduction and oxidation.
Organic PCs – cyanoarenes, benzophenones, quinones, pyryliums, etc. – are well covered in review published by Nicewicz et al. in 2016.1 There were sporadic reports documented earlier in metal PCs, but this concept was revolutionised with key publications from MacMillan et al., Yoon et al. and Stephenson et al.2-4.
Most of the commonly employed visible photocatalysts are polypyridyl complexes of ruthenium and iridium (Figure 2). These complexes provide a unique proposition, with a lower energy of ligand-centered π* orbital in comparison to the metal centered eg orbital. This has benefitted both organic and semiconductor photocatalysis.
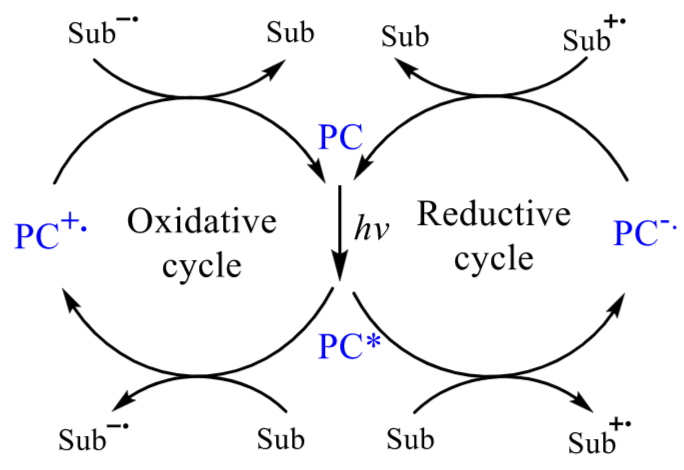
Figure 2 – SET cycle and organometallic catalysts
Scope of chemical conversions
The most promising influence of visible photoredox catalysis is on exceptional bond formations like C(sp3)-C(sp3), C(sp3)-C(sp2) couplings and so on, that are not amenable under conventional conditions, and its ability to operate in redox neutral conditions. Various organic transformations, including oxidation, reductions, cyclisations, dehalogenations, cycloadditions, C-H arylations, trifluoromethylation and decarboxylative couplings, have been investigated to establish its versatility and diversity.
New advances in PCs with ‘dual catalysis’ or metallaphotoredox catalysis by pioneering contribution of MacMillan, Doyle and others in cross-coupling reactions have been reported.5 These successful transformations have made PCs a preferred tool for late-stage functionalisation on advanced drug-like molecules for building focused libraries and guiding SPR modifications to address ADME-tox properties.
Photocatalysis platforms
Despite the tremendous advances in this field, the chemical industry is still conservative in assimilating this technology for scale-up and in industrial settings. However, advanced photoreactors (parallel photoscreening and narrow wavelength platforms) and process intensification methods (i.e. photo-flow reactors) are creating new prospects for PC technology acquisition at commercial scales to make it amenable for process efficiency and safety (Figure 3).
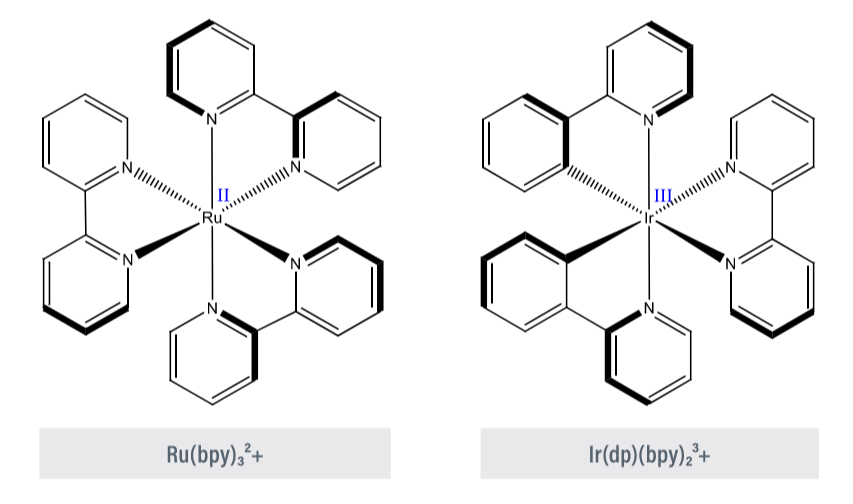
Figure 3 – Technological platforms in photocatalysis
Another key issue is the availability of precious rare-earth metals like ruthenium and iridium, which are costly and limited in the Earth’s crust, and could be subject to supply chain disruption in the future. Nevertheless, the systematic development of organic PCs with computer-aided design is paving the way for greater resilience and effectiveness in PCs.
Application in chemical biology
Photo-catalysis has expanded its horizon in chemical biology applications as well, for instance:
- Photo-uncaging of a microtubule-targeted rigidin analogue in hypoxia cancer cells using green light6
- Photodynamic therapies in cancer treatment7
- C-terminal differentiation for bulk and single molecule proteomics8
- Site-selective tyrosine bioconjugation for native-to-bioorthogonal protein transformation9
- Photocatalysed proximity labelling in a cellular environment under red light10
The MacMillan group has expanded the applicability and potential of iridium photocatalysis through Dexter energy transfer for carbene generation, and subsequent insertion for protein mapping in cellular microenvironment.11
Integration of electro-photochemistry
Other businesses getting benefits from this technology are the material science, water treatment, polymer and solar cell industries. There is a huge opportunity available in integrating photochemistry and electrochemistry. Both are regarded as ‘green’ technologies and participate in oxidative and reductive cycles.
Electrophotochemistry, a concept envisioned by Moutet and Reverdy and demonstrated by Rusling, needs expansion to broaden the scope of reaction paradigm to solve daunting synthetic problems.12,13 Recent reports by Stahl and Wang in merging photochemistry and electrochemistry is a positive step in this direction and will expand the scope of photoredox catalysis.14
A futuristic technology
The increase utility of photoredox catalysis will augment energy conservation, atom economy and waste management efforts. The use of solar energy to drive chemical transformations could be a huge breakthrough in pharmaceutical, agro and fine chemical industry for sustainability and cost efficiency, making it a perfect partner in greener chemistry. More investigation and exploration are required to emulate the functioning of plant leaves, an enormous ubiquitous photoreactors in nature.
CONTACT
Somesh Sharma, PhD
Senior Vice President & Head, Discovery Chemistry Solutions
Aragen Life Sciences Pvt Ltd.
somesh.sharma@aragen.com
www.aragen.com
References
- Nicewicz et al., Chem. Rev., 2016, 116(17), 10075-10166
- MacMillan et al., Science, 2008, 322(5898), 77-80
- Yoon et al., J. Am. Chem. Soc., 2008, 130(39), 12886-122887
- Stephenson et al., J. Am. Chem. Soc., 2009, 131(25), 8756-8757
- Sanford et al., J. Am. Chem. Soc., 2011, 133(46), 18566-18569; Pirnot et al., Science, 2013, 339(6127), 1593-1596; Doyle et al., J. Am. Chem. Soc., 2018, 140(43), 14059-14063; MacMillan et al., Nature, 2018, 560, 70-75
- Bonnet et al., J. Am. Chem. Soc., 2019, 141(46), 18444-18454
- Samantaray et al., ChemBioChem., 2021, 22(23), 3270-3272
- Anslyn et al., ACS Chem. Biol., 2021, 16(11), 2595-2603
- MacMillan et al., Nature Chemistry, 2021, 13, 902-908
- Tay et al., ChemRxiv, 2021
- MacMillan et al., Science, 2020, 367(6482), 1091-1097
- Moutet & Roverdy, Tet. Lett., 1979, 20(26), 2389-2392
- Rusling & Shukla, J. Phys. Chem., 1985, 89(15), 3353-3358
- Stahl &Wang, Angew. Chem. Int. Ed., 2019, 58(19), 6385-6390
Illustrations as supplied:
Figure 1 – Publication counts on photoredox catalysis
Note: Scifinder search, 25 December 2021
Figure 2 – SET cycle & organometallic catalysts
Figure 3 – Technological platforms in photocatalysis
Source: Speciality Chemicals Magazine
