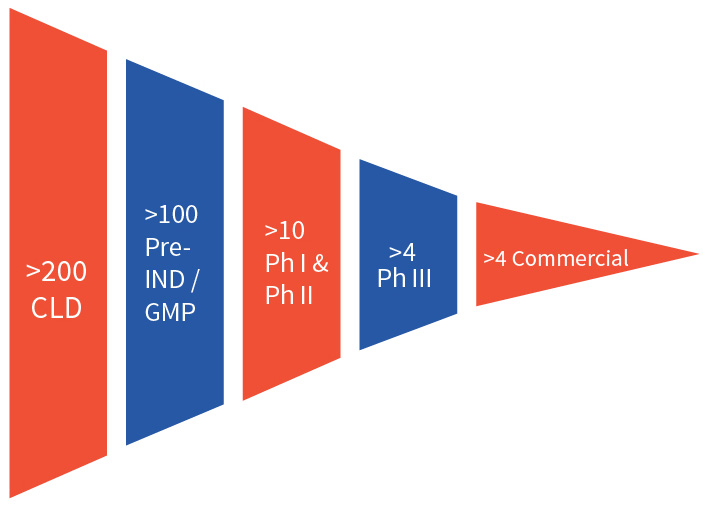
Efficient cell line development is critical in bringing biologics to market and requires a team of experienced scientists, a portfolio of well validated cell line platforms, and state of the art facilities. Aragen delivers this combination and has completed more than 200 cell line development projects, with over 100 of those cell lines in the clinic following an investigational new drug (IND) application. More than four of Aragen’s cell lines are producing marketed products.
Aragen’s researchers are specialists in handling a wide range of host cell lines (CHO, SP2/0, and NS0) and expression vectors (DHFR, Glutamine Synthetase (GS), and antibiotics).
Our Biologics team has produced a range of biologics, including human, mouse, canine and feline IgGs, fusion proteins, enzymes, hormones, cytokines, mini bodies, and bispecific antibodies with this platform. Major advantage of RapTr2022 is best suited to produce difficult-to-express proteins.
A strong track record in successfully engineering cell lines to develop the proprietary CHO-DG44 platform for timely delivery of more than 100 CLD projects. Majority of these CLD projects resulted in IND applications leading to clinical development.
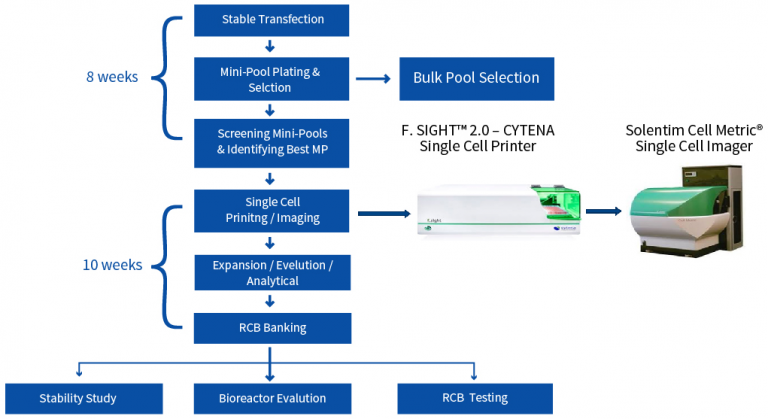
Our resources and capabilities will fit your unique requirements at each step of the CLD process.
Our RapTr2022 platform should expedite your biotherapeutic product journey to clinic since this platform minimizes risk and maximizes efficiency.
CHO DG44Our internal CHO DG44 platform is an essentially free-to-own (royalty-free) and is a high- productivity CHO cell line option that can deliver >4g/L in 5 months for a range of biologics. The DG44 platform has an extensive regulatory track record and uses commercially available media and feeds.
Highlights
CHO GS Platform with Higher titre and shorter Timeline for Antibody Production
Now, increasingly, the GS-CHO expression system, which is protein-free-adapted CHO-K1-derived cell line that employs the glutamine synthetase (GS) gene expression system is used for biotherapeutic production.
Sigma’s CHOZN
Sigma’s CHOZN platform is based on deletion of the CHO glutamine synthetase (GS) gene with Talon gene editing technology. The resulting GS-/- CHO host and expression vector with GS selection is a great combination for companies with an interest in an established GS selection system. Regulators are familiar with its efficacy and operation, and its expression vector produces titers equivalent to the DG44 platform.
Deciding what CLD platform would work best is challenging. Therefore, Aragen’s cell line development service provides cost-effective approaches to test different platforms simultaneously without delaying IND filing timeline.
A poor expressing product is a suitable candidate for testing on multiple platforms. Simultaneous testing provides a mitigation strategy to the risk of having to repeat CLD due to low titers or poor product quality that results in high material costs (COGS). If two platforms have comparable titers, then the superior product quality or reduced milestone costs can drive the platform decision. We have optimized breaks in the CLD process so that parallel work can be stopped as soon as data is available to select the most effective platform.
